New di-anchoring A–π-D–π-A configured organic chromophores for DSSC application: sensitization and co-sensitization studies†
Abstract
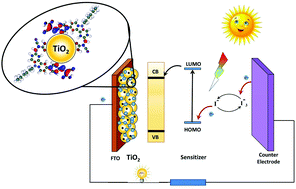
Herein, we report the design and synthesis of three new un-symmetrical metal-free carbazole based organic dyes, E1–3 with A–π-D–π-A architecture, as effective di-anchoring sensitizers in DSSCs. The new entities comprise carbazole as a donor scaffold connected to three different units, viz. cyano acetic acid, 2,4-thiazolidinedione and barbituric acid as acceptor/anchoring units via vinylene and phenylene as π-spacers at 3- and 6-positions of the carbazole ring, respectively. Photophysical, electrochemical and theoretical studies were carried out in order to assess their feasibility as active sensitizers. Furthermore, their photoelectrochemical performances and charge transport properties in fabricated DSSCs were evaluated. The results revealed that the device fabricated with the E1 sensitizer displayed the highest PCE of 2.38% among the three dyes. Its JSC, VOC, and IPCE values were found to be 6.36 mA cm−2, 0.599 V, and 57%, respectively. Its enhanced performance is attributed to the presence of a highly electron withdrawing cyano acetic acid unit on either side of the carbazole core through appropriate π-spacers. Interestingly, the DFT study indicated that the electron cloud of the LUMO level has been shifted significantly towards the 2-cyano phenyl acrylic acid connected at the 6th position of the carbazole ring, when compared to the cyano acrylic acid linked at position 3, confirming efficient charge separation in E1. The assigned lifetimes of E1–3 obtained from EIS studies were found to be in accordance with experimentally obtained photovoltaic parameters. Furthermore, E1–3, when co-sensitized with NCSU-10 sensitizer in DSSCs, displayed higher VOC values, but lower PCE values than that of NCSU-10.


 Please wait while we load your content...
Please wait while we load your content...