Sequential detection of Fe3+/2+ and pyrophosphate by a colorimetric chemosensor in a near-perfect aqueous solution†
Abstract
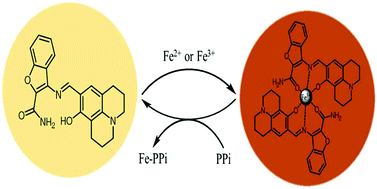
A novel colorimetric chemosensor 1 was designed and synthesized for Fe3+/2+ and pyrophosphate. Sensor 1 showed a selective color change toward both Fe3+ and Fe2+ from yellow to brown in a near-perfect aqueous solution. The detection limits (0.36 μM and 0.37 μM) for Fe3+ and Fe2+ were much lower than the guideline (5.37 μM) set by the Environmental Protection Agency (EPA) for iron in drinking water. Sensor 1 could be used to quantify Fe3+ in real water samples. Moreover, the resulting Fe3+-2·1 complex can detect pyrophosphate selectively over various anions especially including phosphate-based anions through a metal-complex displacement method. Based on UV-vis titrations, Job plot and ESI-mass spectrometry analyses, the sensing mechanisms of Fe3+, Fe2+ and PPi were proposed.


 Please wait while we load your content...
Please wait while we load your content...