Photophysics of 7-mercapto-4-methylcoumarin and derivatives: complementary fluorescence behaviour to 7-hydroxycoumarins†
Abstract
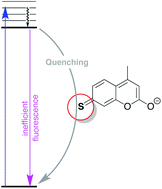
The photophysical behaviour of 7-mercapto-4-methylcoumarin (C-SH) and derivatives has been studied in different solvents. In contrast to 7-hydroxy-4-methylcoumarin, C-SH shows poor emission, but high fluorescence when the thiol is alkylated. The origin and character of the lowest singlet states are discussed, specifically proposing that the thione-like C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S resonance form plays a key role in excited state deactivation in C-SH.
S resonance form plays a key role in excited state deactivation in C-SH.


 Please wait while we load your content...
Please wait while we load your content...