Proton-coupled charge-transfer reactions and photoacidity of N,N-dimethyl-3-arylpropan-1-ammonium chloride salts†
Abstract
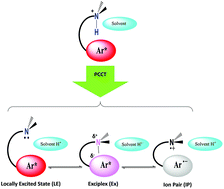
Photoinduced intermolecular proton-transfer processes from N,N-dimethyl-3-arylpropan-1-ammonium chloride salts (ArCl, with aryl as 1-pyrenyl, 9-anthryl, and 2-naphtyl) to a solvent molecule have been investigated by steady-state and dynamic spectroscopic methods. The intermolecular proton-transfers are coupled either to the formation of an exciplex or to a solvent-separated ion pair in what we have termed a ‘proton-coupled charge-transfer reaction’. A range of solvents has been observed to mediate both the ground-state conformations of the ArCl and the extent of electron transfer. Unlike typical photoacids, in which through-bond interactions control photoacidity, through-space charge-transfer interactions are responsible in the excited singlet states of the ArCl. Transient absorption experiments reveal a range of electronic comportments in the excited-states of the ArCl. Excited-state pKa values of −3.4, 1.3, and −3.3 in THF were calculated using a Förster-like approach for the 1-pyrenyl, 9-anthryl, and 2-naphthyl salts, respectively. The observed rate of proton-transfer was found to be independent of the thermodynamic driving force and the short-term reversibility of these reactions has been approximated. The data suggest how other systems may be designed to facilitate this novel process.


 Please wait while we load your content...
Please wait while we load your content...