Systematic investigations on fused π-system compounds of seven benzene rings prepared by photocyclization of diphenanthrylethenes†
Abstract
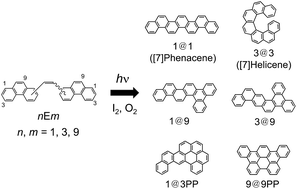
We studied the photoproducts of 1-(n-phenanthryl)-2-(m-phenanthryl)ethenes (nEm; n, m = 1, 3 and 9) for understanding photocyclization patterns based on NMR spectroscopy. The crystal structures of the photoproducts were analyzed by X-ray crystallography, and the photophysical features of the photocyclized molecules were investigated based on emission and transient absorption measurements. Phenanthrene derivatives substituted at the 1- and 3-positions were prepared for synthesizing nEm by photocyclization of stilbene derivatives. We obtained four types of primary photoproducts (n@m) from the corresponding nEm. Two of them were found to have racemic molecular structures in the single crystal determined by X-ray crystallography. Besides the primary photoproducts, two types of secondary photoproducts (n@mPP) were isolated. Fluorescence quantum yields and lifetimes of the obtained photoproducts were determined in solution whereas the definite fluorescence quantum yields were obtained in the powder. Observation of the triplet–triplet absorption spectra in solution by laser photolysis techniques showed that intersystem crossing to the triplet state competes with the fluorescence process.


 Please wait while we load your content...
Please wait while we load your content...