Role of Cr(iii) deposition during the photocatalytic transformation of hexavalent chromium and citric acid over commercial TiO2 samples†
Abstract
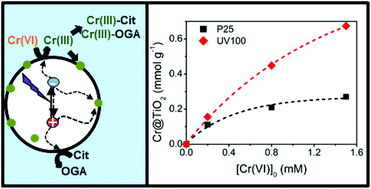
Removal of Cr(VI) and citric acid (Cit) by heterogeneous photocatalytic Cr(VI) transformation under UV light over two commercial TiO2 samples (1 g L−1), Evonik P25 and Hombikat UV100, was studied at pH 2 and Cr(VI) concentrations between 0.2 and 3 mM, with a fixed [Cit]0/[Cr(VI)]0 molar ratio (MR) of 2.5. In both cases, up to complete Cr(VI) removal, the temporal profiles of Cr(VI) and Cit were well adjusted to a pseudo-first order rate law with the same rate constant, evidencing that Cr(VI) removal controls the kinetics of the system. Once Cr(VI) is fully removed, Cit degradation continues with a Langmuir–Hinshelwood behaviour. In all cases, the rate constants decreased with increasing [Cr(VI)]0, and time resolved microwave conductivity (TRMC) measurements revealed that this was due to an increasing retention of Cr(III) on the surface of the photocatalysts, which reduces the lifetime of the electrons. Both kinetic experiments and TRMC measurements confirm that UV100 is not only more efficient than P25 for Cr(VI) and Cit removal, but it is also less influenced by the poisoning of the surface, consistent with its larger specific area. The use of Cit as the sacrificial agent improves the rate and efficiency of the photocatalytic Cr(VI) removal, and also the stability of the photocatalyst by preventing Cr(III) deposition, due to the formation of soluble Cr(III)-complexes, envisaged as a general result of the presence of oligocarboxylic acids in the photocatalytic Cr(VI) system.

 Please wait while we load your content...
Please wait while we load your content...