Application of spectroscopic and theoretical methods in the studies of photoisomerization and photophysical properties of the push–pull styryl-benzimidazole dyes†
Abstract
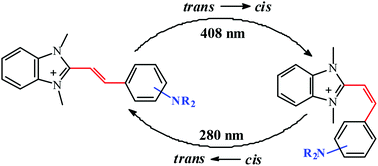
The synthesis and spectroscopic properties of a series of substituted 1,3-dimethyl-2-aminostyrylbenzimidazolium iodides are described and discussed. The products were identified by NMR, IR and UV-Vis spectroscopy and elemental analysis. Their electronic absorption and fluorescence band positions are affected by the character of the substituent and by the solvent polarity. The fluorescence decay of the dyes shows two lifetimes interpreted in terms of emission from two forms of the dye in the excited state. Moreover, the photochemical trans → cis isomerization is reported for these compounds. It occurs from the first excited singlet state of the trans isomer to the cis isomer following a trans-S0 → S1 excitation. The electron-donating character of the substituent in a styrene moiety is one of the crucial factors influencing the photoisomerization process. The structure of the cis isomer was established by 1H and 15N NMR. Experimental studies are supported by the results of quantum chemical calculations.

 Please wait while we load your content...
Please wait while we load your content...