π-Extension of a 4-ethoxy-1,3-thiazole via aryl alkyne cross coupling: synthesis and exploration of the electronic structure†
Abstract
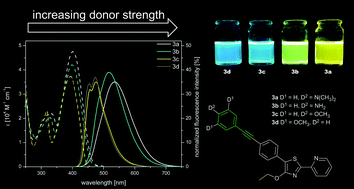
A series of four donor aryl alkynyl substituted thiazole derivatives 3a–d and three similar aryl donor–acceptor systems 6a–c have been synthesized. All compounds bear different electron-donating groups in the 5-position of the thiazole core. The influence of both electron donor strength and the additional phenylethynyl unit on photophysical properties, i.e. UV/Vis absorption, fluorescence emission and fluorescence lifetime, has been evaluated. Additionally, theoretical calculations have been performed at the CAM-B3LYP/6-31+G(d,p) level and good agreement with the experimental data has been achieved. The new derivatives synthesized via palladium catalyzed cross coupling are characterised by moderately strong emission between 474 and 538 nm (ΦF = 0.35–0.39) and Stokes’ shifts ranging from 0.54 to 0.79 eV (4392–6351 cm−1). The smaller chromophores of type 6 exhibit modest to high fluorescence emission (ΦF = 0.45–0.76) between 470 and 529 nm and their Stokes’ shifts range from 0.59 to 0.65 eV (4765–5251 cm−1).

 Please wait while we load your content...
Please wait while we load your content...