Structural and functional roles of the N- and C-terminal extended modules in channelrhodopsin-1
Abstract
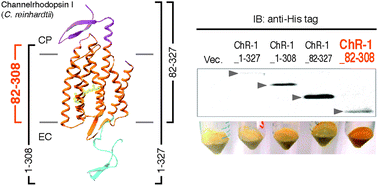
Channelrhodopsins have become a focus of interest because of their ability to control neural activity by light, used in a technology called optogenetics. The channelrhodopsin in the eukaryote Chlamydomonas reinhardtii (CrChR-1) is a light-gated cation channel responsible for motility changes upon photo-illumination and a member of the membrane-embedded retinal protein family. Recent crystal structure analysis revealed that CrChR-1 has unique extended modules both at its N- and C-termini compared to other microbial retinal proteins. This study reports the first successful expression of a ChR-1 variant in Escherichia coli as a holoprotein: the ChR-1 variant lacking both the N- and C-termini (CrChR-1_82-308). However, compared to ChR-1 having the extended modules (CrChR-1_1-357), truncation of the termini greatly altered the absorption maximum and photochemical properties, including the pKa values of its charged residues around the chromophore, the reaction rates in the photocycle and the photo-induced ion channeling activity. The results of some experiments regarding ion transport activity suggest that CrChR-1_82-308 has a proton channeling activity even in the dark. On the basis of these results, we discuss the structural and functional roles of the N- and C-terminal extended modules in CrChR-1.
- This article is part of the themed collection: 16th International Conference on Retinal Proteins

 Please wait while we load your content...
Please wait while we load your content...