Influence of a chromophore analogue in the protein cage of a photoactive yellow protein†
Abstract
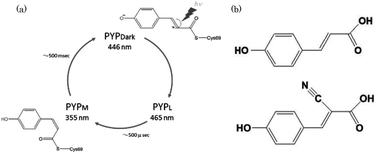
Time-resolved spectra of a photoactive yellow protein (PYP) containing cyano-p-coumaric acid (CHCA) were recorded. To understand the mechanism of photo-isomerization, an electron-withdrawing CN group was introduced into the PYP to alter the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond character. Free CHCA chromophores in aqueous solution underwent photo-isomerization whereas PYP with a bound CHCA (PYP-CN) exhibited no photocycle at acidic or alkaline pH or in urea and other solutions. Furthermore, no photocycle was observed with PYP mutants after illumination. This phenomenon cannot be fully explained by the electron-withdrawing properties of the CN group. We conclude that the CHCA chromophore in PYP was locked in the protein cage and that the CN group interacted with the protein residues.
C double bond character. Free CHCA chromophores in aqueous solution underwent photo-isomerization whereas PYP with a bound CHCA (PYP-CN) exhibited no photocycle at acidic or alkaline pH or in urea and other solutions. Furthermore, no photocycle was observed with PYP mutants after illumination. This phenomenon cannot be fully explained by the electron-withdrawing properties of the CN group. We conclude that the CHCA chromophore in PYP was locked in the protein cage and that the CN group interacted with the protein residues.
- This article is part of the themed collection: 16th International Conference on Retinal Proteins

 Please wait while we load your content...
Please wait while we load your content...