Helical rearrangement of photoactivated rhodopsin in monomeric and dimeric forms probed by high-angle X-ray scattering
Abstract
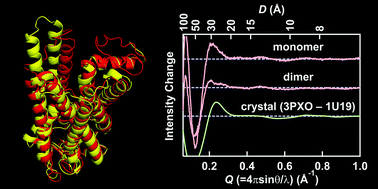
Light-induced helical rearrangement of vertebrate visual rhodopsin was directly monitored by high-angle X-ray scattering (HAXS), ranging from Q (= 4π sin θ/λ) = 0.03 Å−1 to Q = 1.5 Å−1. HAXS of nanodiscs containing a single rhodopsin molecule was performed before and after photoactivation of rhodopsin. The intensity difference curve obtained by HAXS agreed with that calculated from the crystal structure of dark state rhodopsin and metarhodopsin II, indicating that the conformational change of monomeric rhodopsin in the membrane is consistent with that occurring in the crystal. On the other hand, the HAXS intensity difference curve of nanodiscs containing two rhodopsin molecules was significantly reduced, similar to that calculated from the crystal structure of the deprotonated intermediate, without a large conformational change. These results suggest that rhodopsin is dimerized in the membrane and that the interaction between rhodopsin molecules modulates structural changes.
- This article is part of the themed collection: 16th International Conference on Retinal Proteins

 Please wait while we load your content...
Please wait while we load your content...