Solvent viscosity influence on the chemiexcitation efficiency of inter and intramolecular chemiluminescence systems
Abstract
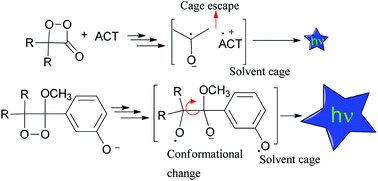
The effects of the medium viscosity on the chemiexcitation quantum yields of the induced decomposition of 1,2-dioxetanes (highly efficient intramolecular CIEEL system) and the catalyzed decomposition of diphenoyl peroxide and a 1,2-dioxetanone derivative (model systems for the intermolecular CIEEL mechanism, despite their low efficiency) are compared in this work. Quantum yields of the rubrene catalyzed decomposition of diphenoyl peroxide and spiro-adamantyl-1,2-dioxetanone as well as the fluoride induced decomposition of a phenoxy-substituted 1,2-dioxetane derivative are shown to depend on the composition of the binary solvent mixture toluene/diphenyl ether, which possess similar polarity parameters but different viscosities. Correlations of the quantum yield data with the medium viscosity using the diffusional and the frictional (free-volume) models indicate that the induced 1,2-dioxetane decomposition indeed occurs by an entirely intramolecular process and the low efficiency of the intermolecular chemiluminescence systems (catalyzed decomposition of diphenoyl peroxide and 1,2-dioxetanone derivative) is not primarily due to the cage escape of radical ion species.

 Please wait while we load your content...
Please wait while we load your content...