Lophine derivatives as activators in peroxyoxalate chemiluminescence†
Abstract
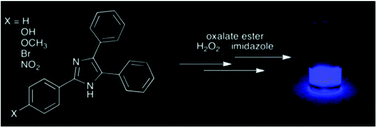
Lophine and four of its derivatives were used as activators (ACTs) of the chemiluminescent peroxyoxalate (PO) reaction of bis(2,4,6-trichlorophenyl)oxalate with H2O2, catalysed by imidazole. Kinetic emission assays have shown that with the tested compounds the reaction mechanism, regarding the formation of the high energy intermediate (HEI) of the PO reaction, occurs as previously seen for commonly used ACTs. A bimolecular interaction of the compounds with the HEI leads to chemiexcitation through the chemically initiated electron exchange luminescence (CIEEL) mechanism, as confirmed by a linear free-energy correlation between the relative catalytic rate constants and the oxidation potentials of the compounds. The yields of excited state formation and light emission, in the range of 10−2–10−3 E mol−1, are comparable to the ones seen with commonly used ACTs. A Hammett plot with ρ = −0.90 indicates the buildup of a partial positive charge on the transition step of the catalytic process, consistent with the formation of a radical cation of the ACT, being an additional validation of the CIEEL mechanism in this system.

 Please wait while we load your content...
Please wait while we load your content...