Abstract
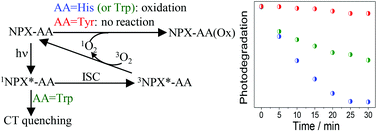
The photooxygenation of model compounds containing the two enantiomers of naproxen (NPX) covalently linked to histidine (His), tryptophan (Trp) and tyrosine (Tyr) has been investigated by steady state irradiation, fluorescence spectroscopy and laser flash photolysis. The NPX–His systems presented the highest oxygen-mediated photoreactivity. Their fluorescence spectra matched that of isolated NPX and showed a clear quenching by oxygen, leading to a diminished production of the NPX triplet excited state (3NPX*–His). Analysis of the NPX–His and NPX–Trp photolysates by UPLC-MS–MS revealed in both cases the formation of two photoproducts, arising from the reaction of singlet oxygen (1O2) with the amino acid moiety. The most remarkable feature of NPX–Trp systems was a fast and stereoselective intramolecular fluorescence quenching, which prevented the efficient formation of 3NPX*–Trp, thus explaining their lower reactivity towards photooxygenation. Finally, the NPX–Tyr systems were nearly unreactive and exhibited photophysical properties essentially coincident with those of the parent NPX. Overall, these results point to a type II photooxygenation mechanism, triggered by generation of 1O2 from the 3NPX* chromophore.
- This article is part of the themed collection: Dedicated to the memory of Prof. Nicholas J. Turro

 Please wait while we load your content...
Please wait while we load your content...