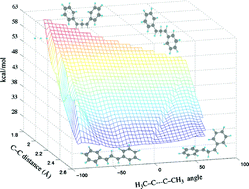
Radical ion pairs generated by photo-induced electron transfer from 1,2-disubstituted cyclopropanes to various acceptors undergo return electron transfer in pairs of singlet and triplet multiplicity. The pair energies relative to the reactant ground states and to accessible triplet states, respectively, determine the competition between the recombination pathways. The potential surfaces of the radical cations and triplet states of 1,2-diphenyl-, 1, and 1,2-dimethylcyclopropane, 2, have been examined by density functional theory calculations. The radical cation surfaces have minima at geometries that retain significant bonding between C-1 and C-2, preventing geometric isomerization of the radical cations. The triplet potential surfaces are dissociative with minimal rotational differentiation at long distances between C-1 and C-2.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...