Control of pyrene fluorescence intensity by in situ addition of CO2 to an amidine/amine mixture or CO2 removal from an amidinium carbamate ionic liquid†
Abstract
Addition of CO2 to an equimolar
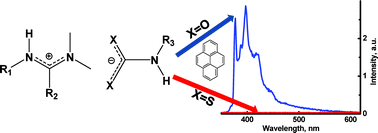
- This article is part of the themed collection: In honour of Jean-Pierre Desvergne

 Please wait while we load your content...
Please wait while we load your content...