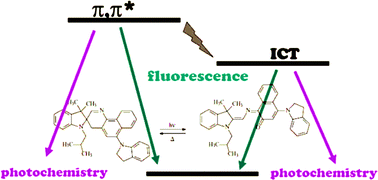
A great variety of technological applications makes photochromism a currently appealing theme for basic studies. In this work, excited state dynamics of two spirooxazines and two naphthopyrans, that upon UV irradiation undergo thermally reversible conversion to coloured photomerocyanines, have been investigated by using pump–probe techniques (femtosecond time resolution). The breakage of the C–O bond, involved in the photoreaction, has been found to occur within a few hundreds of femtoseconds producing a first transient that evolved on picosecond time-scale to the most stable isomer through a number of intermediates that depended on the solvent and the structure of the photochrome. The peculiar behaviour of one of the molecules studied (1,3-dihydro-3,3-dimethyl-1-isobutyl-6′-(2,3-dihydro-1H-indol-1-yl)spiro [2H-indole-2,3′-3H-naphtho[2,1-b][1,4]oxazine]) has been investigated in depth in various media because it revealed an unusual dual photochemistry pathway. This finding is traced to reactivity of π,π* and ICT excited states whose relative populations are controlled by the polarity of the solvent.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...