Time-dependent UV/visible absorption changes are observed on photolysis of aerated solutions of monodisperse, vinyl end-capped 2,5-diheptyloxyl substituted PV-oligomers (OPV, n = 1, 2, 3). In all cases, photolysis occurs in at least two distinct stages. Evidence for the intermediacy of singlet oxygen comes from pulse radiolysis and time-resolved luminescence studies, which confirm formation of the OPV triplet state and its quenching by molecular oxygen to produce singlet oxygen. The subsequent reaction with oligomers is observed directly by time-resolved phosphorescence and indirectly through product identification. The results strongly suggest that the reaction between singlet oxygen and OPV proceeds via cycloaddition, leading to bond scission and aldehyde group formation. The reactivity of vinylene linkages is greater than of vinyl end groups. Consequently, the photostability decreases as the chain length is extended. Implications on the oxidative degradation of poly(p-phenylenevinylene)s in optoelectronic devices, and of competing singlet oxygen and superoxide radical anion degradation pathways, are discussed.
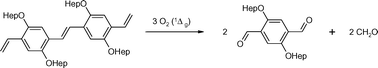
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
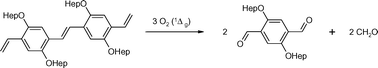

 Please wait while we load your content...
Please wait while we load your content...