A novel elsinochrome A derivative: a study of drug delivery and photodynamic activity
Abstract
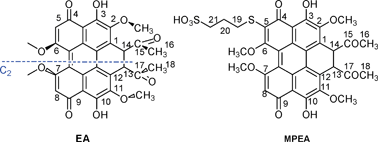
Elsinochrome A (EA) possesses the highest singlet-oxygen quantum yield (0.98) amongst the perilenoquinoid pigments and may be suitable as a phototherapeutic drug. However, there have been virtually no studies into its medicinal applications. Based on the analysis of chemical derivatives of hypocrellins (the same family as EA), 5-(3-mercapto-1-propanesulfonic acid)-substituted elsinochrome A (MPEA) with an amphiphilicity was designed and synthesized by considering drug delivery and biological activity requirements. MPEA possesses a water solubility of 5.1 mg mL−1, which is just sufficient to enable dissolution at a clinically acceptable concentration, while its partition coefficient (n-octanol/phosphate buffered saline) of 7 guarantees affinity to biological targets. MPEA could photogenerate semiquinone anion radicals and reactive oxygen species, especially singlet oxygen, at a yield of 0.73, which approaches that for hypocrellin B. Biological tests confirmed that the photodynamic activity of MPEA was as high as 60% of that of its parent EA, which is significantly higher than that of most other photosensitizers.

 Please wait while we load your content...
Please wait while we load your content...