Recombinant Metridia luciferase isoforms: expression, refolding and applicability for in vitro assay
Abstract
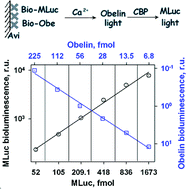
The recombinant coelenterazine-dependent luciferases (isoforms MLuc164 and MLuc39) from the marine copepod Metridia longa were expressed as inclusion bodies in E. colicells, dissolved in 6 M

 Please wait while we load your content...
Please wait while we load your content...