Self-aggregation of synthetic zinc chlorophyll derivatives possessing multi-perfluoroalkyl chains in perfluorinated solvents†
Abstract
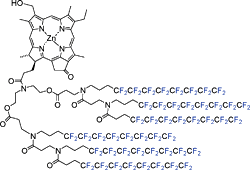
Zinc 31-hydroxy-131-oxo-chlorins possessing two, three, four and six perfluorooctyl chains were synthesized from naturally occurring chlorophyll-a. Only the synthetic zinc chlorin possessing six perfluorooctyl chains was directly dissolved in perfluorinated ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C131, which was confirmed from resonance Raman spectral analysis.
C131, which was confirmed from resonance Raman spectral analysis.

 Please wait while we load your content...
Please wait while we load your content...