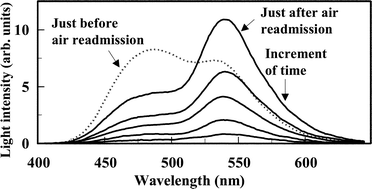
To elucidate the reversible change in the color of bioluminescence (BL) arising from Vibrio fischeri Y1, the relationship between the BL color and the redox state of endogenous yellow fluorescent protein (YFP), carrying riboflavin 5′-phosphate (FMN), has been investigated in vitro. YFP lost fluorescence with a maximum at 538 nm when reduced, and retrieved its original fluorescence upon reoxidation. Such a change in YFP fluorescence was analogous to that of free FMN. In the NADH/FMN oxidoreductase-coupled luciferase reaction with YFP, yellow BL peaking around 535 nm was largely depressed when sodium dithionite was added. This phenomenon can be attributed to the reduction of YFP; i.e., reduced YFP does not participate in the luciferase reaction as a secondary emitter. On admitting air into the reaction mixture, the yellow light characteristic of V. fischeri Y1 BL was regenerated. These results indicate that the reversible change in YFP fluorescence is caused by the redox change of YFP-bound FMN, and that the change in BL color between blue and yellow is associated with the redox state of YFP.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...