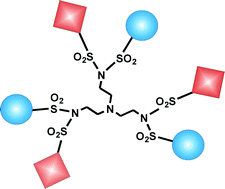
We report the photophysical properties (absorption and emission spectra, quantum yield, and lifetime) of five dendrimers of first generation based on a TREN (tris(2-aminoethyl)amine) skeleton functionalized at the periphery with naphthyl and/or 5-dimethylamino-1-naphthalenesulfonamide (hereafter called dansyl) chromophores. Each dendrimer comprises one tertiary amine unit in the core and three branches carrying a sulfonimido unit at the periphery, each one substituted by two identical or different moieties. In particular, TD6 and TN6 contain dansyl (D) or naphthyl (N) units, respectively, while TD3B3, TN3B3 and TN3D3 contain dansyl, naphthyl or benzyl (B) units at the periphery. The spectroscopic behaviour of these dendrimers has been investigated in acetonitrile solution and compared with that of reference compounds. For all dendrimers the absorption bands are red shifted compared to those of monomeric naphthyl and dansyl reference compounds. Moreover, the intense naphthyl and dansyl fluorescence is greatly quenched because of strong interactions between the two aromatic moieties linked by a sulfonimido unit. Protonation of the amine units of the dendrimers by addition of CF3SO3H (triflic) acid causes a decrease in intensity of the luminescence and a change in the shape of the emission bands. The shapes of the titration curves depend on the dendrimer, but in any case the effect of acid can be fully reversed by successive addition of base (tributylamine). The obtained results reveal that among the intradendrimer interactions the most important one is that taking place (via mesomeric interaction) between the various chromophores and a pair of sulfonimido groups.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...