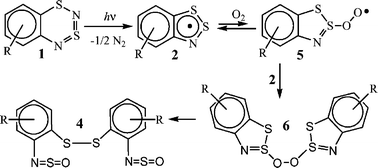
Photochemistry of 1,3,2,4-benzodithiadiazines: formation and oxidation of 1,2,3-benzodithiazolyl radicals
Abstract
Photolysis of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-substituted diphenyl disulfides via a self termination-like process with an effective second-order rate constant depending linearly on the concentration of dissolved O2.
N-substituted diphenyl disulfides via a self termination-like process with an effective second-order rate constant depending linearly on the concentration of dissolved O2.

 Please wait while we load your content...
Please wait while we load your content...