Synthetic and photochemical studies of substituted 1-acyl-7-nitroindolines†
Abstract
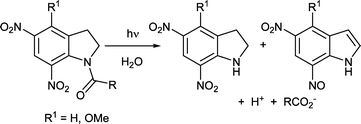
A previous study of substituent effects on the photocleavage of 1-acyl-7-nitroindolines has been extended to examine the effects of electron-donating and electron-withdrawing substituents. 1-Acetyl-4,5-methylenedioxy-7-nitroindoline 7 was inert to 350 nm irradiation, reinforcing an earlier finding that excessive electron-donation by substituents can divert the excited state into non-productive pathways. By contrast, the 1-acetyl-5,7-dinitro- and 1-acetyl-4-methoxy-5,7-dinitroindolines 8 and 9 respectively both showed improved photolysis efficiency in aqueous solution compared to the 1-acyl-4-methoxy-7-nitro compound 2. Unlike 2, both 8 and 9 gave mixed photoproducts, the corresponding dinitroindolines and the 5-nitro-7-nitrosoindoles. These results are interpreted in terms of a previous mechanistic study. Investigation of the 4-methoxy-5,7-dinitroindoline conjugate of L-glutamate 18 showed that the stoichiometry of

 Please wait while we load your content...
Please wait while we load your content...