A role of the C-terminal extension of the photosystem II D1 protein in sensitivity of the cyanobacterium Synechocystis PCC 6803 to photoinhibition†
Abstract
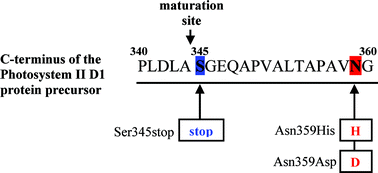
The D1
- This article is part of the themed collection: In honour of James Barber

 Please wait while we load your content...
Please wait while we load your content...