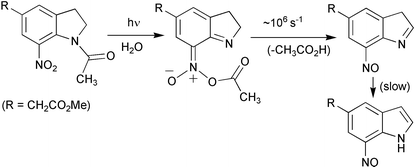
1-Acyl-7-nitroindolines have been found to be useful photoactivated protecting groups for rapid release of carboxylates in aqueous solution. Mechanistic details of carboxylic acid photorelease from model compounds in solutions of varying H2O–CH3CN composition are now reported, using data from product studies, deuterium isotope effects, kinetic studies (UV-Vis), and nanosecond laser flash photolysis. Our data support a mechanism (via T1) that involves reaction of a critical photogenerated intermediate 21
(an acetic nitronic anhydride), obtained via photochemical transfer of the acetyl group from the amide nitrogen to one of the oxygen atoms of the nitro group. This mode of transfer has been reported in the photochemistry of a variety of N-acetyl-o-nitrodiphenylamines. Two competing pathways of reaction from 21 that differ in how the acyl group is cleaved are proposed to account for the products observed. In solutions of higher water content, the predominant reaction pathway of 21 is via an AAL1-like cleavage that results in formal intramolecular redox reaction of the aromatic ring system, to give the released carboxylic acid and 7-nitrosoindole 3
(after tautomerisation of an initially formed nitroso-3H-indole 9). In solutions of low water content, the major pathway for hydrolysis of 21 is via the standard addition–elimination mechanism (AAC2) with water as the nucleophile, that releases the carboxylic acid and nitroindoline 4. Laser flash photolysis studies in wholly aqueous medium gave a transient (within the 20 ns laser pulse) at λmax 450 nm, assignable to 21. Single exponential decay of this species in water (kobs
= 5 × 106 s−1) is assigned to the release of the carboxylic acid and formation of the nitroso-3H-indole 9, which is supported by time-resolved measurements of acid release using bromothymol blue. Therefore, 1-acyl-7-nitroindolines photorelease their protected functionalities at rates in the submicrosecond time scale.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...