Synthesis and second order nonlinear optical properties of new chromophores containing 1,3,4-oxadiazole and thiophene rings
Abstract
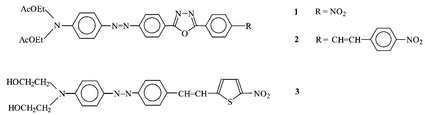
We report the synthesis and physico-chemical characterization, including molecular second order nonlinear optical properties by the EFISH technique, of three new heterocycle based push–pull

 Please wait while we load your content...
Please wait while we load your content...