Theoretical study on the reaction between 4,6-dimethyl-1,2,3-triazine and enamines
Abstract
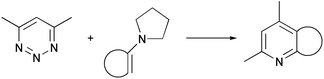
The reaction between 4,6-dimethyl-1,2,3-triazine and cyclopentanone pyrrolidine enamine has been studied using ab initio SCF-MO computational methods. Solvent effects have also been taken into account. The reaction is predicted to be a concerted Diels–Alder cycloaddition. Self-consistent reaction field methods based on multipole expansions of the free energy of solvation tend to overestimate the stability of zwitterionic intermediates at the Hartree–Fock level. This overestimation results in a stepwise mechanism, although higher levels of theory predict a concerted mechanism.

 Please wait while we load your content...
Please wait while we load your content...