The pH dependence of the anisotropy factors of essential amino acids†
Abstract
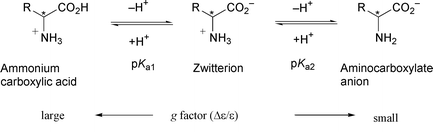
In order to examine the pH dependence of the anisotropy factor (g) of all the essential amino acids, at pHs 1, 2, 7 and 11, the molar absorption coefficient (ε) and circular dichroism (Δε) were determined in aqueous solution. The g factors were high, and except for Asn and the aromatic and sulfur-containing compounds (Tyr, His, Trp, Cys and Met) the gmax values are larger than 0.01 in the pH range 1–7. Large g factors of aliphatic amino acids are observed even at pH 11. On the whole the magnitudes of the g factors at pH 1 are 2–3 times those at pH 7. The n,π* transition of the carboxylic group is the major contributor to the large g factors in amino acids such as Ala and Val. It is thought that in connection with the possible origin of amino acid homochirality generated by circularly polarised light irradiation, the % ee (≡ % op) of the amino acid generated will also be pH dependent in a manner similar to that previously reported for Leu.

 Please wait while we load your content...
Please wait while we load your content...