Metal ion-promoted cleavage of mRNA 5′-cap models: hydrolysis of the triphosphate bridge and reactions of the N7-methylguanine base
Abstract
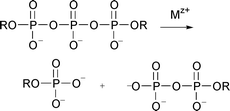
Reactions of mRNA 5′-cap model compounds were studied to evaluate the potential of these reactions in the development of artificial RNases. Diadenosine triphosphate was used as a model for the triphosphate bridge, and its hydrolysis was studied in the presence of several Cu2+ complexes. The results of the kinetic experiments show that bifunctional catalysis by phosphate bound Cu2+ complexes is involved. The most efficient catalysis is achieved with complexes with acidic aqua ligands, and a metal ion-bound hydroxo ligand most probably acts as a nucleophile in the reaction. A detailed mechanism cannot, however, be suggested on the basis of the data. N7-methylguanosine and its 5′-monophosphate and diphosphate were used to study the reactions of the N7-methylguanine base of the mRNA 5′-cap moiety. While Cu2+ complexes efficiently enhance the hydrolysis of the triphosphate bridge, little effect on the reactions of the N7-methylguanine base was observed: neither the cleavage of the imidazole ring or the depurination of the nucleoside were enhanced to any significant extent.

 Please wait while we load your content...
Please wait while we load your content...