Thermodynamic, spectroscopic, and density functional theory studies of allylaryl and prop-1-enyl aryl ethers. Part 1. Thermodynamic data of isomerization
Abstract
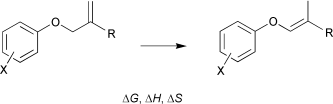
A chemical equilibration study of the relative thermodynamic stabilities of seventy isomeric allyl aryl ethers (a) and (Z)-prop-1-enyl aryl ethers (b) in DMSO solution has been carried out. From the variation of the equilibrium constant with temperature the Gibbs energies, enthalpies, and entropies of isomerization at 298.15 K have been evaluated. Because of their low enthalpies, the (Z)-prop-1-enyl aryl ethers are strongly favored at equilibrium, the Gibbs energies of the a→b isomerization ranging from −12 to −23 kJ mol−1. The entropy contribution is negligible in most reactions, but occasionally small positive values less than +10 J K−1 mol−1 of the entropy of isomerization are found. The equilibration studies were also extended to involve two pairs of related isomeric

 Please wait while we load your content...
Please wait while we load your content...