Structure of the dimers arising from one-electron electrochemical reduction of pyridinium salts 3,5-disubstituted with electron-withdrawing groups
Abstract
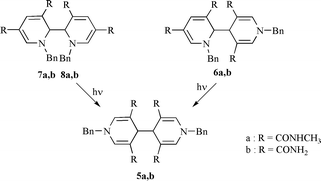
One-electron electrochemical reduction of the salts 1-benzyl-3,5-bis(methylcarbamoyl)pyridinium bromide 3a and 1-benzyl-3,5-dicarbamoylpyridinium bromide 3b yields mixtures of four isomeric dimers, as shown by HPLC analysis and mass spectrometry. 1H and 13C NMR spectrometry allows us to determine the structures of the mixture components. In both mixtures, the two most abundant products are identified as 4,4′- and 2,4′-tetrahydrobipyridines (5a, 5b and 6a, 6b, respectively), while minor amounts of a pair of diastereomeric 2,2′-linked dimers are also detected in (7a, 8a and 7b, 8b respectively). Therefore, the NMR studies lead to the conclusion that the structure assignment of conformers of 5a, made previously for 6a and 7a, is not correct. All the 2,2′- and 2,4′-linked dimers undergo photochemical dissociation into two pyridinyl radicals which recombine to yield 4,4′-linked dimers.

 Please wait while we load your content...
Please wait while we load your content...