N- and O-Alkylation of isoquinolin-1-ones in the Mitsunobu reaction: development of potential drug delivery systems
Abstract
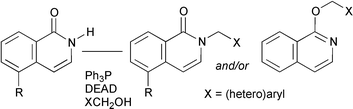
Regioselective methods were investigated to prepare N- and O-alkylated isoquinolin-1-ones efficiently. The predicted regioselective

 Please wait while we load your content...
Please wait while we load your content...