Synthetic studies toward the kempane diterpenes. Approaches to the assembly of the ring system
Abstract
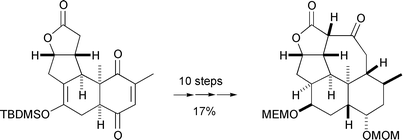
The ring system of the kempane diterpenes has been assembled from the Diels–Alder adduct 7 by a highly chemo- and stereoselective attack of lithium ethoxyacetylide on its apparently more encumbered carbonyl (to give 11), removal of the silyl protecting group (12 and 13), concomitant deoxygenation and ethoxyethyne solvolysis (18 and 19), reconjugation and epimerization (21), and then a series of reductive and protection steps before cyclization of the final seven-membered ring (31). An alternative approach is outlined which was thwarted by an unusual cyclization of a dimethyl ether moiety (48) to a tetrahydrofuran (49).

 Please wait while we load your content...
Please wait while we load your content...