Synthesis of biphenylenes and tetraphenylenes using copper-catalyzed coupling of arylzinc intermediates
Abstract
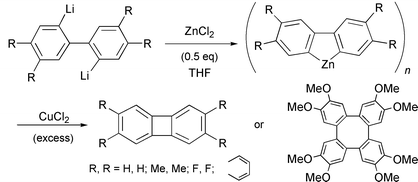
Biphenylene and some of its 2,3,6,7- and 1,8-substituted derivatives were synthesized using the CuCl2-mediated intramolecular coupling of an organozinc species prepared from 2,2′-dilithiobiaryls with one or two molar equiv. of ZnCl2 or ZnBr2 in THF. Although most of the reactions of 2,2′-dilithiobiaryls with CuCl2 in THF in the absence of ZnCl2 or ZnBr2 led to biphenylenes as a major product, similar reactions of the organozinc species with CuCl2 in THF produced biphenylenes in much better yields, due to smooth transmetallation and reductive elimination reactions. In particular, the copper-mediated cyclization of benzannelated organozinc intermediates, prepared from equimolar proportions of 2,2′-dilithiobiaryls with ZnCl2, proceeded smoothly and selectively to afford the desired biphenylenes in 46–81% yield except for the reaction of the zinc intermediate derived from 4,4′,5,5′-tetramethoxy-2,2′-dilithiobiphenyl with ZnCl2 (1.0 molar equiv.). The reaction of the tetramethoxy-substituted organozinc species with CuCl2 produced 2,3,6,7,10,11,14,15-octamethoxytetraphenylene as a major product in 67% yield.

 Please wait while we load your content...
Please wait while we load your content...