Electrophile promoted cyclization of ortho-aryl substituted ynamides: construction of 3-amino-4-halo- or 4 seleno-isocoumarin derivatives†
Abstract
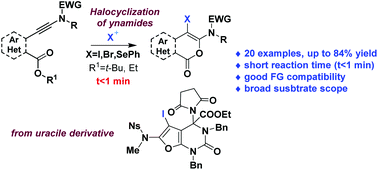
Isocoumarins are important building blocks in medicinal chemistry. They are widespread in the core structure of biologically active compounds. Here we report the development of an efficient and highly reactive electrophilic cyclization of ortho-ynamidyl benzoate esters providing access to 3-amino-4-halo- or 4-seleno-isocoumarins in a short time (<1 min) with good yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...