Nickel-catalyzed reductive 1,3-diene formation from the cross-coupling of vinyl bromides†
Abstract
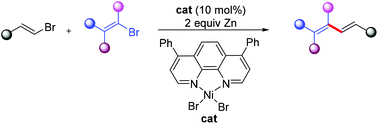
Facile construction of 1,3-dienes building upon cross-electrophile coupling of two open-chain vinyl halides is disclosed in this work, showing moderate chemoselectivities between the terminal bromoalkenes and internal vinyl bromides. The present method is mild and tolerates a range of functional groups and can be applied to the total synthesis of a tobacco fragrance solanone.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...