Formation of amidino-borate derivatives by a multi-component reaction†
Abstract
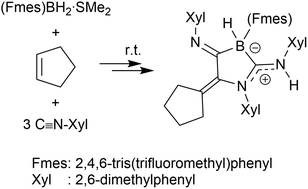
Cyclohexene reacts with the (Fmes)BH2·SMe2 borane reagent and three molar equivalents of the isonitrile CN-Xyl to give the five membered 1,3-BN heterocyclic product 7 that contains a zwitterionic borata-amidinium moiety and a cyclohexenyl substituent. The analogous five-component coupling between cyclopentene, (Fmes)BH2·SMe2 and CN-Xyl in a 1 : 1 : 3 molar ratio gives the related cyclic amidino-borate derivative 10. The reaction of the (Fmes)BH2 derived frustrated Lewis pair 12, in situ generated or employed as the isolated dimer, reacts with 3 CN-Xyl equivs. at elevated temperature (60 °C) to yield the analogous coupling product 13.
- This article is part of the themed collection: p-Block Lewis Acids in Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...