An amphiphilic B,O-chelated aza-BODIPY dye: synthesis, pH-sensitivity, and aggregation behaviour in a H2O/DMSO mixed solvent†
Abstract
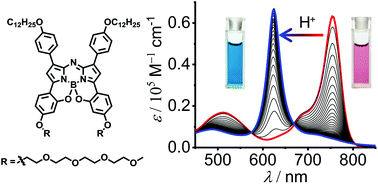
A novel amphiphilic B,O-chelated azadipyrromethene (aza-BODIPY) dye, containing hydrophobic dodecyloxy groups and hydrophilic tetraethylene glycol (TEG) chains, was synthesized and characterized by NMR, HRMS, Vis/NIR absorption and fluorescence spectroscopy. The B,O-chelated dye 1 exhibited largely bathochromically shifted NIR absorption and fluorescence spectra in comparison with common BF2-chelated aza-BODIPY dyes. Upon gradual addition of trifluoroacetic acid (TFA) to the dye 1 solution, obvious spectral changes were observed in Vis/NIR absorption and fluorescence spectroscopy measurements. Meanwhile, the colour change of the dye 1 solution from pink to blue was noticeable by the naked eye, indicating the pH-sensitivity of dye 1. The pH-sensitivity of dye 1 under acidic conditions could be ascribed to the formation of dye species 2·H+. Furthermore, owing to the amphiphilic feature of dye 1, it self-assembled into J-type aggregates in a mixed solvent of water/DMSO (2/8, v/v). Temperature-dependent Vis/NIR spectroscopic studies revealed a cooperative aggregation process of dye 1 and a nanowire-like morphology of the nanoaggregates was observed by AFM.


 Please wait while we load your content...
Please wait while we load your content...