Zinc salt-catalyzed reduction of α-aryl imino esters, diketones and phenylacetylenes with water as hydrogen source†
Abstract
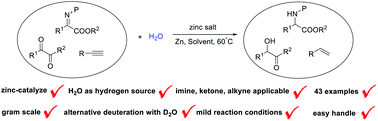
The zinc salt-catalyzed reduction of α-aryl imino esters, diketones and phenylacetylenes with water as hydrogen source and zinc as reductant was successfully conducted. The presented method provides a low-cost, environmentally friendly and practical preparation of α-aryl amino esters, α-hydroxyketones and phenylethylenes. By using D2O as deuterium source, the corresponding products were obtained in high efficiency with excellent deuterium incorporation rate, which gives a cheap and safe tool for access to valuable deuterium-labelled compounds.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...