Selective photoredox direct arylations of aryl bromides in water in a microfluidic reactor†
Abstract
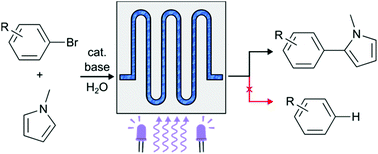
Carrying out photoredox direct arylation couplings between aryl halides and aryls in aqueous solutions of surfactants enables unprecedented selectivity with respect to the competing dehalogenation process, thanks to the partition coefficient of the selected sacrificial base. The use of a microfluidic reactor dramatically improves the reaction time, without eroding the yields and selectivity. The design of a metal free sensitizer, which also acts as the surfactant, sizeably improves the overall sustainability of arylation reactions and obviates the need for troublesome purification from traces of metal catalysts. The generality of the method is investigated over a range of halides carrying a selection of electron withdrawing and electron donating substituents.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...