Radical bromination-induced ipso cyclization–ortho cyclization sequence of N-hydroxylethyl-N-arylpropiolamides†
Abstract
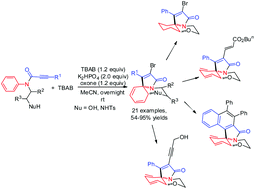
A facile procedure is reported for the synthesis of various 2-bromo-1-phenyl-5,6-dihydro-3H,7aH-benzo[b]pyrrolo[2,1-c][1,4]oxazin-3-ones via a radical bromination-induced ipso cyclization–ortho cyclization sequence of N-arylpropiolamides in the presence of TBAB and oxone. The radical cyclization sequence involves a radical bromo α-addition into the alkyne, ipso-cyclization, and ortho-trapping of the spirocyclic intermediate.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...