Exploring the synthetic potential of a marine transaminase including discrimination at a remote stereocentre†
Abstract
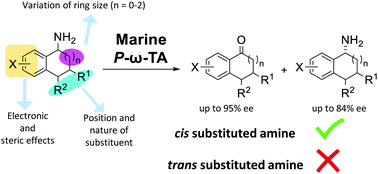
The marine transaminase, P-ω-TA, can be employed for the transamination from 1-aminotetralins and 1-aminoindanes with differentiation of stereochemistry at both the site of reaction and at a remote stereocentre resulting in formation of ketone products with up to 93% ee. While 4-substituents are tolerated on the tetralin core, the presence of 3- or 8-substituents is not tolerated by the transaminase. In general P-ω-TA shows capacity for remote diastereoselectivity, although both the stereoselectivity and efficiency are dependent on the specific substrate structure. Optimum efficiency and selectivity are seen with 4-haloaryl-1-aminotetralins and 3-haloaryl-1-aminoindanes, which may be associated with the marine origin of this enzyme.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...