Lewis acid-catalyzed double addition of indoles to ketones: synthesis of bis(indolyl)methanes with all-carbon quaternary centers†
Abstract
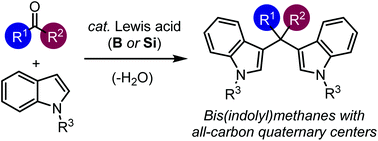
We report herein a Lewis acid-catalyzed nucleophilic double-addition of indoles to ketones under mild conditions. This process occurs with various ketones ranging from dialkyl ketones to diaryl ketones, thereby providing access to an array of bis(indolyl)methanes bearing all-carbon quaternary centers, including tetra-aryl carbon centers. The products can be transformed into bis(indole)-fused polycyclics and bis(indolyl)alkenes.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...