Burchellin and its stereoisomers: total synthesis, structural elucidation and antiviral activity†
Abstract
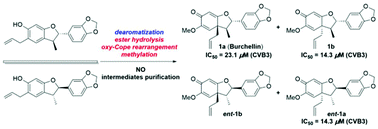
Burchellin and its analogues are a class of neolignan natural products containing a rare core with three contiguous stereogenic centers. In previous reports, racemic burchellin was synthesized without accessing each of the enantiomers. In this paper, a concise and efficient total synthetic route to divergently access the enantiomers of burchellin and those of its 1′-epi-diastereoisomer over six steps for each is disclosed, where each of the enantiomers was obtained by preparative chiral phase HPLC purification. The key steps include the construction of a 2,3-dihydrobenzofuran moiety by two Claisen rearrangements and a one-step rearrangement/cyclization and subsequent tandem ester hydrolysis/oxy–Cope rearrangement/methylation to furnish the basic skeleton of burchellin. The structures and absolute configurations of the four stereoisomers were determined using spectroscopic data analyses and comparison of experimental and calculated electronic circular dichroism data. These stereoisomers were found to have potent antiviral effects against coxsackie virus B3, and is the first time that bioactivity has been reported for these compounds.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...