Metal-free benzoylation of imidazoheterocycles by oxidative decarboxylation of arylglyoxylic acids†
Abstract
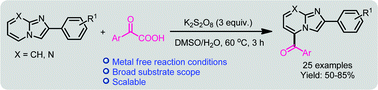
A simple and straightforward approach has been realized for the direct benzoylation of imidazoheterocycles by oxidative decarboxylation of arylglyoxylic acids in the presence of K2S2O8 as an oxidant. Various functional groups were tolerated on both imidazoheterocycles and arylglyoxylic acids and a wide range of C5-benzoyl-imidazoheterocycles were obtained in good to high yields (50–84%). Radical trapping experiments confirmed the involvement of the radical pathway. The developed protocol is amenable for a scale-up reaction.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...