Total synthesis of the actinoallolides and a designed photoaffinity probe for target identification†
Abstract
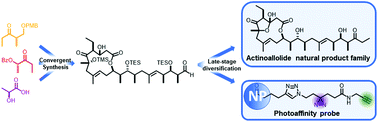
The actinoallolides are a family of polyketide natural products isolated from the bacterium Actinoallomurus fulvus. They show potent biological activity against trypanosomes, the causative agents of the neglected tropical diseases human African trypanosomiasis (sleeping sickness) and Chagas disease, while exhibiting no cytotoxicity against human cell lines. Herein, we give a full account of our strategy evolution towards the synthesis of this structurally unique class of 12-membered macrolides, which culminated in the first total synthesis of (+)-actinoallolide A in 20 steps and 8% overall yield. Subsequent late-stage diversification then provided ready access to the congeneric (+)-actinoallolides B–E. Enabled by this flexible and efficient endgame sequence, we also describe the design and synthesis of a photoaffinity probe based on actinoallolide A to investigate its biological mode of action. This will allow ongoing labelling studies to identify their protein binding target(s).
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...