Rhodium-catalyzed synthesis of substituted isoquinolones via a selective decarbonylation/alkyne insertion cascade of phthalimides†
Abstract
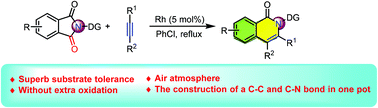
A rhodium-catalyzed decarbonylation/alkyne insertion cascade of phthalimides has been established. The reaction can be carried out in an operationally simple manner and provides expedient access to a series of isoquinolones in moderate to good yields. This reaction proceeded through a sequential decarbonylation/alkyne insertion/intramolecular annulation procedure and featured good functional group tolerance, ample substrate scope, and the construction of C–C and C–N bonds in one pot.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...