Oxovanadium-catalysed domino reactions of hydroxy enynes for the construction of Cashmeran-like odorants†
Abstract
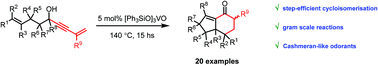
An atom-economical oxovanadate-catalysed cycloisomerization of hydroxy enynes for the synthesis of bicyclo[4.3.0]non-1(9)-en-2-ones is disclosed, which can be rationalised through a cascade reaction of a dissociative Meyer–Schuster rearrangement to allenyl vanadates, followed by a thermal intramolecular Diels–Alder (IMDA) reaction and hydrolytic regeneration of the catalyst.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...